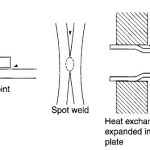
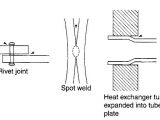
A brief Definition of corrosion
10 May 2020A brief Definition of corrosion
Corrosion is generally taken to be the wastage of a metal by the action of corrosive agents. However, a wider definition is the degradation of a material through contact with its environment. Thus corrosion can include non-metallic materials such as concrete and plastics and mechanisms such as cracking in addition to wastage (i.e. loss of material).
In essence, the corrosion of metals is an electron transfer reaction. An uncharged metal atom loses one or more electrons and becomes a charged metal ion.
In an ionizing solvent the metal ion initially goes into solution but may then undergo a secondary reaction, combining with other ions present in the environment to form an insoluble molecular species such as rust or aluminium oxide. In high-temperature oxidation the metal ion becomes part of the lattice of the oxide formed.
More Posts:
Design implication for corrosion behaviour
Plant Structure Corrosion Monitoring